Bioquell ProteQ
The latest achievement from Bioquell, the Bioquell ProteQ offers a modular approach to room and zone decontamination. Enhanced distribution capabilities, networking options, wireless technology and advanced aeration capabilities make this system, the ideal solution for contamination elimination in the smallest labs to the largest production areas.
Idéal pour:
- Domaines de fabrication biopharmaceutique
- Laboratoires de production
- Décontamination des salles et des zones
- Laboratoires de biosécurité
- Salles blanches
- Laboratoires BPF/BPL

WHY CHOOSE THE Bioquell ProteQ
Rapid
Capable to reduce cycles times significantly; use in existing facilities without complex modifications

Integrated
One piece of equipment performs vaporization and aeration stages; additional aeration units stored within the frame of the equipment

Compliant
Thermal and PDF printouts of key cycle data; 21 CFR Part 11 compliant audit trail available
Productive
Deploy the system anywhere in a facility or campus; ability to run parametric cycles for quick decontamination of new areas
Efficient
Enhanced distribution technology; faster cycle times; reduce consumable usage

Easy to Use
Intuitive graphical user-interface; ability to store and run 1000s of predefined cycles; wireless connectivity allows rapid setup and reduced downtime


WATCH THE BIOQUELL PROTEQ VIDEO
Preview key benefits and features
CONFIGURATION

BIOQUELL PROTEQ COMPONENTS





ACCESSORIES

Additional Aeration Units
Additional aeration units can be added to the Bioquell ProteQ and Bioquell BQ-50 to disperse vapor, as well as provide further aeration, resulting in faster cycles. These units connect wirelessly with the system. Up to 2 aeration units can be stored within the frame of the Bioquell ProteQ for ease of transport.

Additional aeration units can be added to the Bioquell ProteQ and Bioquell BQ-50 to disperse vapor, as well as provide further aeration, resulting in faster cycles. These units connect wirelessly with the system. Up to 2 aeration units can be stored within the frame of the Bioquell ProteQ for ease of transport.
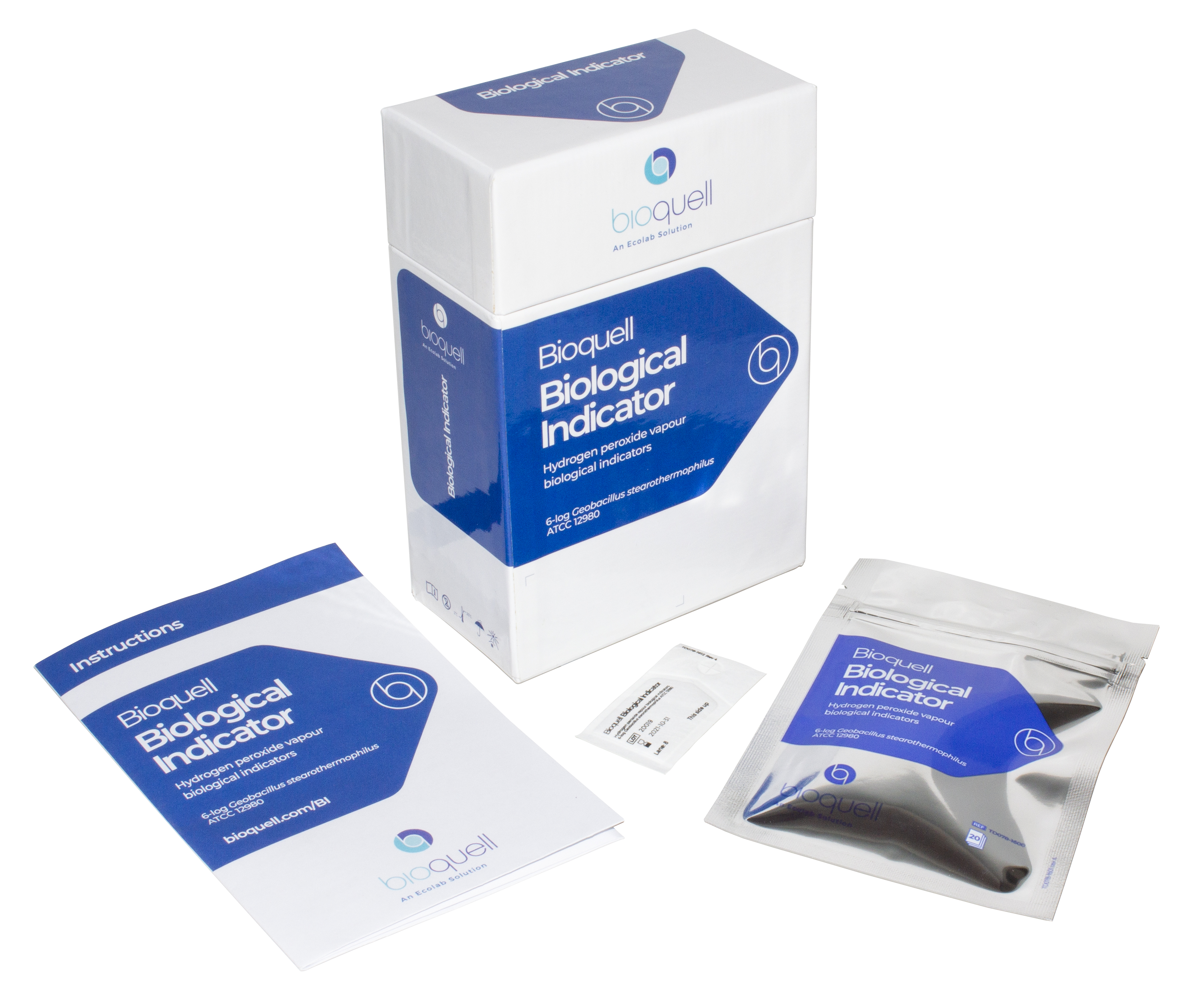
Our individually crafted biological indicators made from Geobacillus stearothermophilus endospores ensure reliable results when seeking to validate a 6-log reduction of bioburden from the decontamination process

Low Level Peroxide Sensor XAM-5100
This peroxide sensor is a safety tool used to evaluate the levels of hydrogen peroxide remaining upon the completion of a cycle to ensure the area is safe to enter. It provides an added level of reassurance beyond the readings provided by the Bioquell system.

The Bioquell Room CI is our chemical indicator developed for rooms 25m3 and larger. Place one or multiple indicators in the room you are performing Bioquell bio-decontamination to see your expected results in real-time. The color changing strip printed on the CI has a reactive dye specifically tailored for monitoring Bioquell’s bio-decontamination process, allowing you to see the results as they occur.
VALIDATION
Our specialist team of validation engineers execute the IQ (Installation Qualification), OQ (Operational Qualification) and PQ (Performance Qualification) protocols of each new Bioquell ProteQ.
With the Bioquell ProteQ’s ease of use and capacity to decontaminate nearly any area in your facility, validation can be as simple or complex as you require. Bioquell will support you in the development and proof of optimized cycle parameters to achieve repeatable kill results in each specific room and load configurations. Bioquell can perform the work for you or train your team in how to create, monitor and adjust programs.
FAQs
Are aeration units within the system included?
Each Bioquell ProteQ unit features an integrated aeration unit for the removal of hydrogen peroxide vapour. Additional mobile aeration units are not included, allowing you to optimise the system to your individual requirements as extra aeration capabilities may not be needed.
How large a room can the Bioquell ProteQ decontaminate?
The Bioquell ProteQ can support a room up to 400m3. This is subject to configuration, loading, and environmental conditions. If needed, larger spaces can be decontaminated with additional Bioquell ProteQ systems.
Can the Bioquell ProteQ control my HVAC?
The system can communicate with your BMS via volt-free contacts to indicate cycle progress. The BMS can then control your HVAC accordingly.
What if the wireless connectivity does not work in my building?
While extremely rare, if this situation occurs, we can supply a wireless range extender to allow for a wired connection when needed.
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.










